van der waals forces
Johannes Diderik van der Waals Dutch pronunciation. In a symmetrical molecule like hydrogen however there doesnt seem to.
 |
| Torn Crystal Reveals Interlayer Van Der Waals Forces Research Chemistry World |
Gaya Van der Waals merupakan gaya tarik menarik listrik yang relatif lemah akibat kepolaran molekul yang permanen atau terinduksi.

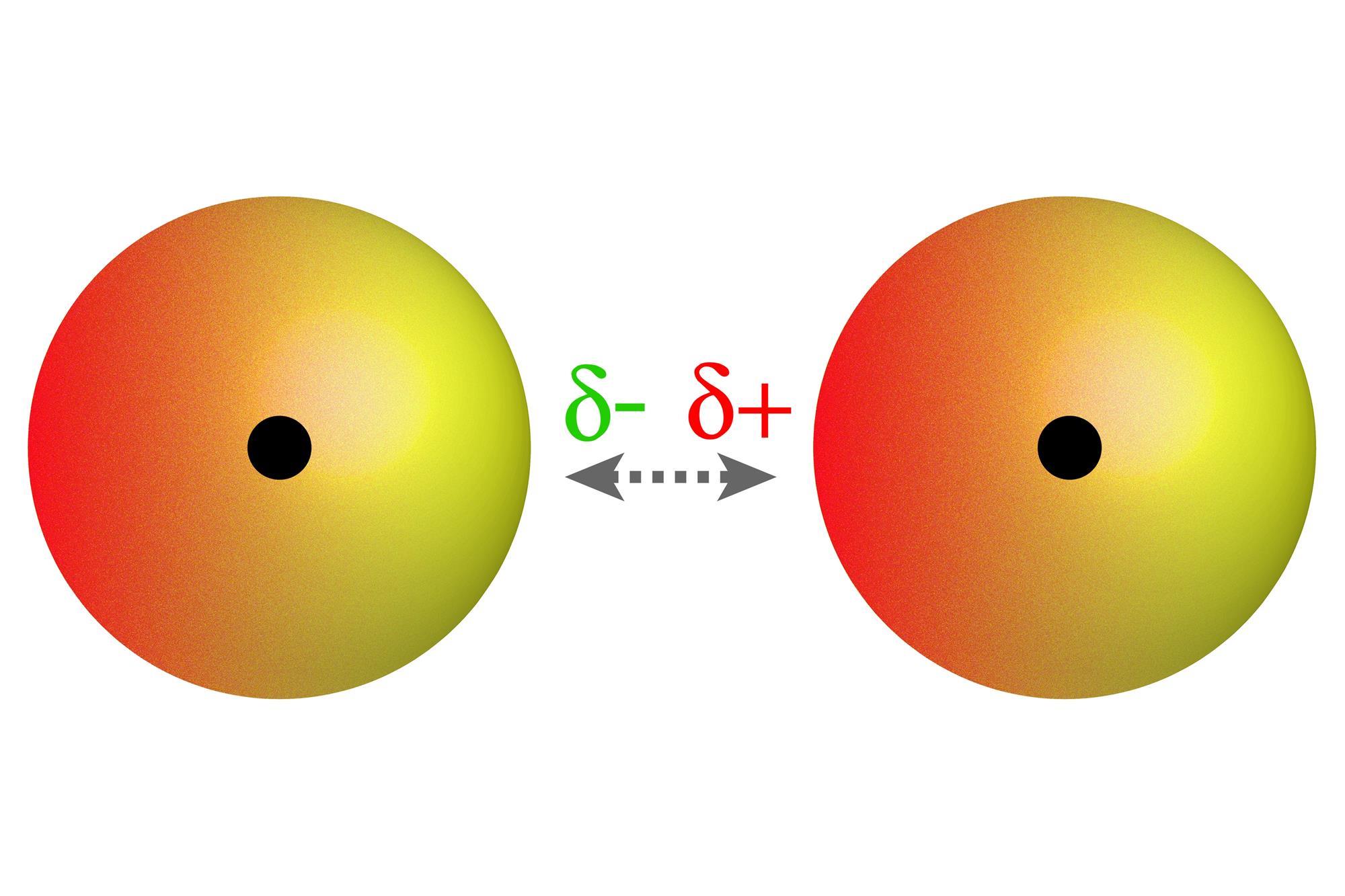
. Van der Waals forces are weak electrostatic forces that attract neutral molecules to one another. The origin of van der Waals dispersion forces Temporary fluctuating dipoles Attractions are electrical in nature. This diagram shows how a whole lattice. I will teach you the basic concepts of van der waals force and different types of van der waals forc.
For Employees of hospitals schools universities and libraries. Van der Waals forces plural noun ˈvan-dər-ˌwȯlz- ˈvän-dər-ˌvälz-. Characteristics of van der Waals. Particles in liquid or air vibrate and move constantly.
The Van der Waals equation is a thermodynamic equation of state based on the theory that fluids are composed of particles with non-zero volumes and subject to a not necessarily pairwise. Van Der Waalss forces of attraction are the weakest among the weak chemical forces having a strength that ranges between 04 and 4-kilo Joulesmol. This single-step material-to-device integration is achieved by decoupling the forces inducing the 2D material transfer from the van der Waals forces at the interface of interest. The van der Waals force is a distance-dependent interaction between atoms or molecules in molecular physics named after Dutch physicist Johannes Diderik van der Waals.
However they may assist a necessary. Thus they collide with other. Kepolaran permanen terjadi akibat kepolaran di dalam. Joːˈɦɑnəz ˈdidərɪk fɑn dər ˈʋaːls.
They are also called dispersion forces. This lecture is about van der waals forces in chemistry. Van der Waals forces are a group of interactions between atoms andor within individual molecules that take place based upon the interaction of electron clouds surrounding two polar. Download up to 8 FREE medical animations from Nucleus by signing up for a free trial at.
Van der Waals forces are weak forces between molecules that occur due to either temporary or permanent dipoles. The Van Der Waals forces between two atoms are affected by a number of factors including the distance between the atoms the nature of the atoms involved and the environment around the. The strength of van der Waals force typically lies between 04 kJmole and 4 kJmole and they tend to act over a distance less than or equal to 04 nm. There are two types of van der Waals forces.
23 November 1837 8 March 1923 was a Dutch theoretical physicist and thermodynamicist. As long as the molecules are close together this synchronized movement of the electrons can occur over huge numbers of molecules. Instantaneous temporary dipole induced dipole forces also called London dispersion forces Permanent dipole permanent. Van der Waals forces aka Van der Waals interactions are the weakest intermolecular force and consist of weak dipole-dipole forces and stronger London dispersion forces.
The relatively weak attractive forces that act on neutral atoms and molecules and that arise because of the electric.
 |
| What Is The Difference Between Van Der Waals Forces And Intermolecular Forces Quora |
 |
| Learn About Van Der Waals Forces In Dna Structure Chegg Com |
 |
| Van Der Waals Forces 1 3 14 Cie As Chemistry Revision Notes 2022 Save My Exams |
 |
| Hydrogen Bonding And Van Der Waals Forces |

Posting Komentar untuk "van der waals forces"